ISOLUTE® Myco SPE Column
multiple mycotoxin sample preparation
Ordering Information
| Part Number |
Description |
Quantity |
| 150-0006-BGK |
ISOLUTE Myco 60 mg/3 mL column (tabless) |
50 |
| PPM-48 |
Biotage PRESSURE+ 48 Positive Pressure Manifold 48 Well |
1 |
| 121-1016 |
VacMaster-10 Sample Processing Manifold complete with 16 mm collection rack |
1 |
| 121-2016 |
VacMaster-20 Sample Processing Manifold complete with 16 mm collection rack |
1 |
| 415000 |
TurboVap LV |
1 |
|
|
|
ISOLUTE® Myco SPE columns offer simple and efficient multiple mycotoxin sample preparation from a wide range of matrices, ideally suited for selective and fast LC-MS/MS analysis. ISOLUTE Myco SPE columns contain a novel polymer-based sorbent(Patent pending) designed specifically to be selective enough to isolate a wide variety of different mycotoxins. One product with multiple applications, simplifying and streamlining your mycotoxin analysis procedures.
Features and Benefits
- Each method confidently meets the maximum residue limits (MRL) set down by EU and US regulations in terms of detection limits, %RSD and recoveries, in most cases far exceeding these requirements.
- SOLUTE Myco clean up methods are quick, simple to use and require no offline steps; making the technique suitable for automation, especially useful for high throughput testing laboratories.
- Unlike immunoaffinity columns, ISOLUTE Myco columns are cost effective, easy to use and have no special storage requirements.
Mycotoxins Quantified Using ISOLUTE Myco
- aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, ochratoxin A, fumonisin B1, T-2 toxin, HT-2 toxin, zearalenone, deoxynivalenol, ergocornine, a-ergocryptine, patulin.
Matrices Tested Using ISOLUTE Myco
- grain (wheat flour, wheat, maize, barley), apple juice, nuts (Brazil nuts), spice (chili).
| Sample Matix |
Analyte Tested |
| Grain (wheat, maize, barley) |
aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, ochratoxin A, fumonisin B1 T-2 toxin, HT-2 toxin, zearalenone, Deoxynivalenol, ergocornine a-ergocryptine |
| Apple Juice |
Patulin |
| Nuts (Brazil nuts) |
aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, ochratoxin A, fumonisin B1 T-2 toxin, HT-2 toxin, zearalenone, Deoxynivalenol, ergocornine a-ergocryptine |
| Spice (Dried chili) |
aflatoxin B1, aflatoxin B2, aflatoxin G1 aflatoxin G2, ochratoxin |
| Infant Formula (Milk) |
aflatoxin M |
| Infant Formula (Cereal) |
aflatoxin B1, aflatoxin B2, aflatoxin G1, aflatoxin G2, ochratoxin A, fumonisin B1, zearalenone, HT-2 mycotoxin, T-2 mycotoxin. | * Applications have been developed using representative analytes from multiple mycotoxin classes, as shown, and may be extended to include other analytes with similar chemical and structural characteristics as required.
* COMMISSION REGULATION (EC) 401/2006 (ii) (laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs).
Mycotoxins are a group of toxic metabolites produced by molds found on food crops. They can enter the food chain either through direct contamination of crops or through livestock feed and then subsequent contamination of farmed meat and dairy products. Their potential to cause harm to humans, crops and farmed animals means that a wide range of food matrices need to be tested for mycotoxin contamination. The diversity of both analyte structure and food matrix combine to produce a unique analytical challenge.
Traditionally, mycotoxin analysis has been performed with difficult to use, expensive immunoaffinity based sample preparation followed by high performance liquid chromatography (HPLC) with UV or fluorescence based detection. Due to the highly specific nature of the immunoaffinity approach, these methods allow determination of individual (or closely related) mycotoxins only, meaning multiple analyses may be required to quantify all the regulated mycotoxins in a particular sample.
Many laboratories would prefer to analyze multiple mycotoxins from each matrix with just one analytical process. The increasing adoption of liquid chromatography-tandem mass spectrometry (LC-MS/MS) based analysis facilitates this multi-analyte approach. Due to the selective nature of LC-MS/MS analysis, mycotoxins, even with similar molecular structures can be resolved at very low levels of detection in one quick assay. Sample preparation and clean up before LC-MS/MS analysis, just like in HPLC testing, is essential for robust, reproducible results, especially when dealing with complex and variable sample types. However traditional sample preparation approaches that can only isolate individual analytes/classes do not match up with this analytical approach.
Unlike more complicated and expensive approaches such as immunoaffinity assays, ISOLUTE Myco employs a simple catch and release solid phase extraction (SPE) procedure to selectively bind mycotoxins of interest to the sorbent, whilst washing off matrix components that could interfere with the analysis. Once interferences have been removed, the mycotoxins of interest can be eluted from the column and quantitatively analyzed by LC-MS/MS. The whole clean up procedure is only eight steps and is fully automation compatible.
Methods developed using ISOLUTE Myco have been designed to extract mycotoxins relevant to each sample matrix according to EU and US regulations.
Each sample type has its own challenges in terms of the interferences present, so matrix specific application notes have been developed to ensure sufficient sample clean up for robust, reliable analysis by LC-MS/MS.
Solid Phase Extraction Using ISOLUTE® Myco SPE Columns
Solid phase extraction is a simple and effective technique used in the extraction of small molecules from complex matrices. Solid food samples are first treated (usually by grinding or homogenization and solvent extraction) to ensure the mycotoxins of interest are freely available in the liquid phase (step 1, below). The ISOLUTE Myco SPE column is prepared for extraction (steps 2 and 3), and the sample is applied. As the liquid sample passes through the SPE column (step 4) mycotoxins are extracted from the sample and adsorbed onto the ISOLUTE Myco sorbent. Unwanted matrix components are then selectively removed from the column using optimized washing procedures (step 5), before the mycotoxins are selectively recovered from the columns using an elution solvent (step 6) resulting in a highly purified extract for analysis.
This ‘catch and release’ procedure using ISOLUTE Myco SPE columns allows the clean-up of a wider range of mycotoxins from different matrices compared with ‘interference removal only’ approaches. 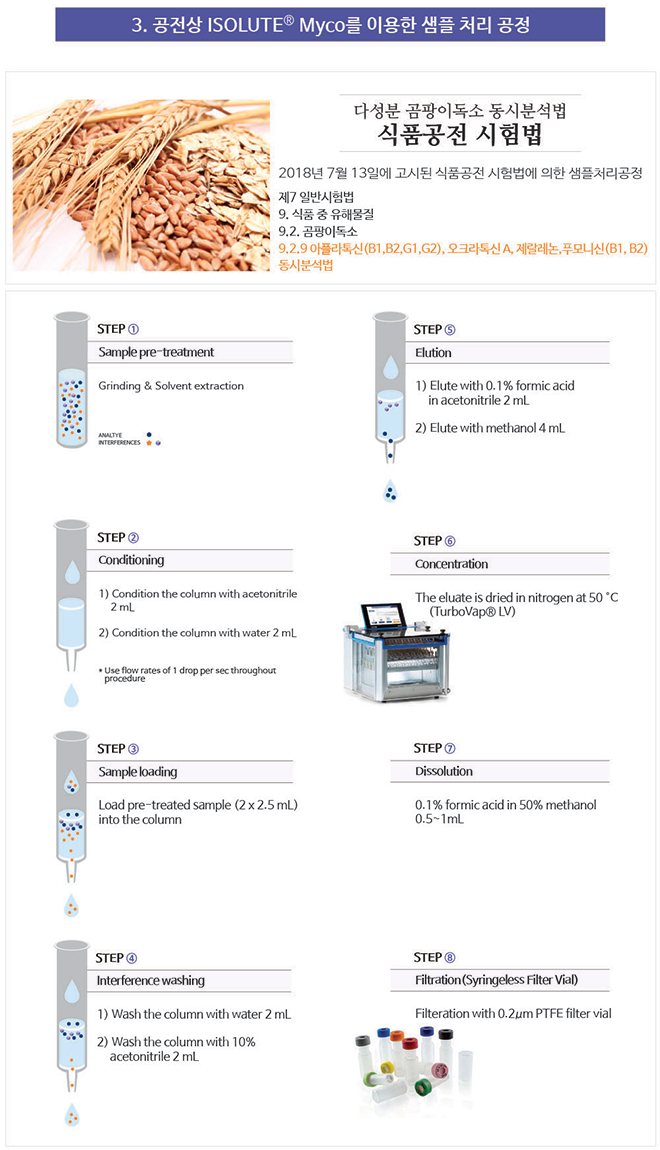
Figure 1. Solid phase extracion procedure using ISOLUTE® Myco columns 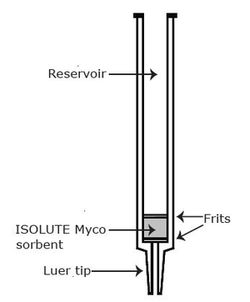 Figure 2. Example of a typical solid phase extraction column Figure 2. Example of a typical solid phase extraction column The ISOLUTE Myco SPE column consists of a tabless cartridge containing 60 mg of mycotoxin specific polymeric sorbent sandwiched between two frits and a standard luer tip for ease of use (see Figure 2). Once conditioned, sample is simply added into the reservoir and allowed to flow through the ISOLUTE Myco sorbent. Mycotoxins are retained on the sorbent, and through a series of simple washes and drying steps any matrix interferences are removed. The mycotoxins are then eluted from the column, for analysis by LC-MS/MS. The ISOLUTE Myco format is compatible with most standard SPE processing equipment, including vacuum and positive pressure manifolds, and automated systems that hold the standard 3 mL column size. |



